
Get the speed and affordability you need, plus powerful customization options not available in competing medical affairs SaaS solutions.
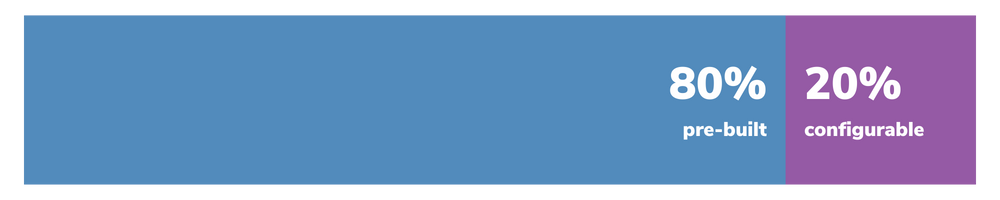
Our use cases offer a perfect balance of pre-built functionality and flexibility, with 80% of the solution already built and the remaining 20% configured to your company's specific requirements.

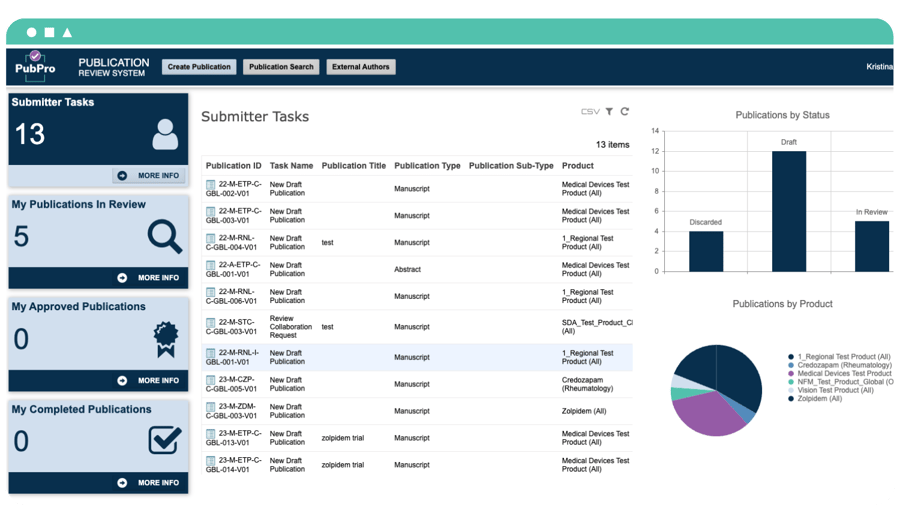
Get new drugs and therapies to market, faster. PubPro helps medical affairs teams cut life sciences publication costs by up to 50% and reduce submission times from hours to minutes.
DOWNLOAD PDF
Publication planning and management is only one piece of a time-consuming drug approval process. The ability to move swiftly is a significant competitive advantage for life sciences companies.
PubPro increases the agility and speed of critical publication workflows so you can get to market faster without compromising the integrity or safety of your products.
Many publication management tools require medical affairs teams to adapt their unique, proprietary processes to fit the rigid confines of one-size-fits-all technology.
PubPro is configured to your unique SOPs, which prevents your users from adopting clunky workarounds or switching between multiple applications.
There is no room for errors or hiccups when potentially life-saving therapies are on the line.
PubPro helps medical affairs teams ensure their publication management processes are air-tight and constantly adapting to ever-changing regulatory and business requirements.
Other competitors |
|
PubPro |
|---|---|---|
| Leaders must manually update assignments for each publication and after team changes | Automated, role-based reviewer assignments | |
| Debarment checks manually performed by medical affairs and legals department | Configurable routing of debarment check results | |
| Complicated dashboards that users must configure themselves | Pre-filtered dashboards, based on user roles assigned at setup | |
| Hard or expensive to customize | Designed specifically to be configurable | |
| Journals and congresses data obtained separately | Real-time integrations of journals and congresses data |
|
|
PubPro |
|---|---|---|
| Leaders must manually update assignments for each publication and after team changes | Automated, role-based reviewer assignments | |
| Debarment checks manually performed by medical affairs and legals department | Configurable routing of debarment check results | |
| Complicated dashboards that users must configure themselves | Pre-filtered dashboards, based on user roles assigned at setup | |
| Hard or expensive to customize | Designed specifically to be configurable | |
| Journals and congresses data obtained separately | Real-time integrations of journals and congresses data |
|
|
PubPro |
|---|---|---|
| Leaders must manually update assignments for each publication and after team changes | Automated, role-based reviewer assignments | |
| Debarment checks manually performed by medical affairs and legals department | Configurable routing of debarment check results | |
| Complicated dashboards that users must configure themselves | Pre-filtered dashboards, based on user roles assigned at setup | |
| Hard or expensive to customize | Designed specifically to be configurable | |
| Journals and congresses data obtained separately | Real-time integrations of journals and congresses data |

Handling medical information requests can be challenging without the right technology. With Process Director, you can centralize MIRs from internal and external stakeholders, including adverse event recordings to HCP dosage questions.
DOWNLOAD PDF
How long does it take for HCPs and patients to receive a response to their medical information request?
Our solution improves the user experience of HCPs and patients by helping you process MIRs faster.
Struggling with tedious, time-consuming report generation due to decentralization of information across multiple data sources?
Compile data from multiple systems and sources so you can quickly generate weekly, quarterly, and yearly reports.
Assuring compliance with regulatory guidance and company-specific policies doesn't need to be an overly complex process.
Our platform's valuable performance insights make it easy to identify bottlenecks, assure compliance, and continuously improve processes.
Our flexible, scalable process automation solutions empower medical affairs teams at life science organizations to operate better and do their best possible work.

With Process Director, you get a process engine that works every time.
There's endless possibilities for automating and optimizing other processes, whether you use one of our pre-built use cases or create your own.
Enjoy the greatest bang for your buck as you create more new processes.
Get the convenience of SaaS with the added luxury of a consultative business partnership. We’re there to support you every step of the way.
It’s no secret that life science organizations must adapt and harness new technologies in order to stay competitive in the market and comply with regulations. Read more in our exclusive white paper.
Download: The Evolving Role of Medical Affairs
